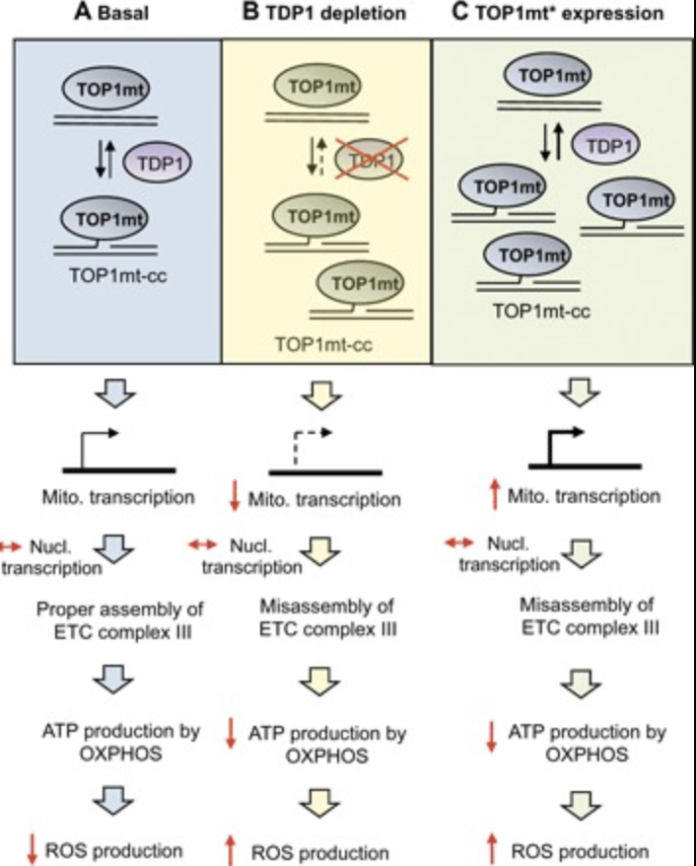
Breakage of one strand of DNA is the most common form of DNA damage. Most damaged DNA termini require end-processing in preparation for ligation. The importance of this step is highlighted by the association of defects in the 3′-end processing enzyme tyrosyl DNA phosphodiesterase 1 (TDP1) and neurodegeneration and by the cytotoxic induction of protein-linked DNA breaks (PDBs) and oxidized nucleic acid intermediates during chemotherapy and radiotherapy. Although much is known about the repair of PDBs in the nucleus, little is known about this process in the mitochondria. We reveal that TDP1 resolves mitochondrial PDBs (mtPDBs), thereby promoting mitochondrial gene transcription. Overexpression of a toxic form of mitochondrial topoisomerase I (TOP1mt*), which generates excessive mtPDBs, results in a TDP1-dependent compensatory up-regulation of mitochondrial gene transcription. In the absence of TDP1, the imbalance in transcription of mitochondrial- and nuclear-encoded electron transport chain (ETC) subunits results in misassembly of ETC complex III. Bioenergetics profiling further reveals that TDP1 promotes oxidative phosphorylation under both basal and high energy demands. It is known that mitochondrial dysfunction results in free radical leakage and nuclear DNA damage; however, the detection of intermediates of radical damage to DNA is yet to be shown. Consequently, we report an increased accumulation of carbon-centered radicals in cells lacking TDP1, using electron spin resonance spectroscopy. Overexpression of the antioxidant enzyme superoxide dismutase 1 (SOD1) reduces carbon-centered adducts and protects TDP1-deficient cells from oxidative stress. Conversely, overexpression of the amyotrophic lateral sclerosis-associated mutant SOD1G93A leads to marked sensitivity. Whereas Tdp1 knockout mice develop normally, overexpression of SOD1G93A suggests early embryonic lethality. Together, our data show that TDP1 resolves mtPDBs, thereby regulating mitochondrial gene transcription and oxygen consumption by oxidative phosphorylation, thus conferring cellular protection against reactive oxygen species-induced damage.
Chiang S-C, Meagher M., Kassouf N., Hafezparast M., McKinnon P.J., Haywood R., El-Khamisy SF. (2017). Mitochondrial protein-linked DNA breaks perturb mitochondrial gene transcription and trigger free radical induced DNA damage. Science Advances. 3(4):e1602506.
Professor of Molecular Medicine, Director of Research and Innovation and co-founder of the Healthy Life Span Institute, University of Sheffield, United Kingdom
Sherif El-Khamisy is a Wellcome Trust Investigator and co-founder of the Healthy Lifespan Institute at the University of Sheffield. El-Khamisy lab studies how cells maintain genomic integrity and their impact on health. The lab uses interdisciplinary approach fusing genetics, chemistry and biology with clinical expertise. We use mouse and zebrafish models to stay ageing and multimorbidity at the molecular and organismal level. We link our molecular understanding to public health challenges through interactions with social scientists.